Which Of The Following Structures Undergoes The Most Physiological Changes During A Menstrual Cycle?

The menstrual bike is a series of natural changes in hormone production and the structures of the uterus and ovaries of the female reproductive system that make pregnancy possible. The ovarian cycle controls the production and release of eggs and the cyclic release of estrogen and progesterone. The uterine bike governs the preparation and maintenance of the lining of the uterus (womb) to receive a fertilized egg. These cycles are concurrent and coordinated, usually last between 21 and 35 days in adult women, with a median length of 28 days, and keep for most 30–45 years.
Naturally occurring hormones drive the cycles; the cyclical rising and autumn of the follicle stimulating hormone prompts the product and growth of oocytes (immature egg cells). The hormone estrogen stimulates the uterus lining to thicken to accommodate an embryo should fertilization occur. The claret supply of the thickened lining (endometrium) provides nutrients to a successfully implanted embryo. If implantation does not occur, the lining breaks down and claret is released. Triggered by falling progesterone levels, menstruation (a "period", in common parlance) is the cyclical shedding of the lining, and is a sign that pregnancy has non occurred.
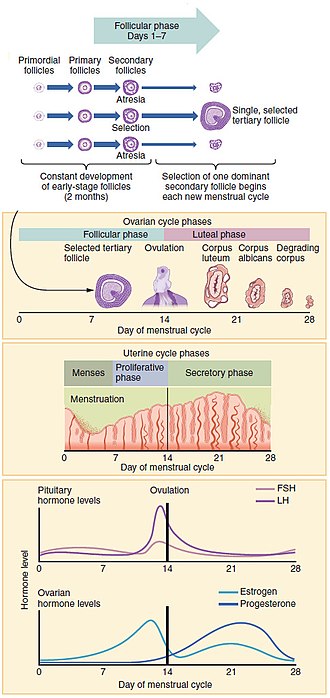
Each cycle occurs in phases based on events in the ovary (ovarian cycle) or the uterus (uterine bike). The ovarian cycle consists of the follicular phase, ovulation, and the luteal stage; the uterine cycle consists of the menstrual, proliferative and secretory phases. Day one of the menstrual wheel is the first day of the period, which lasts for well-nigh five days. Around solar day 14, an egg is normally released from the ovary. Menarche (the onset of the outset menstruum) usually occurs effectually the age of twelve years.
The menstrual bike can cause some women to experience problems that disrupt daily lives. These can include cramps, tender breasts, tiredness, and premenstrual syndrome. More than severe problems such as premenstrual dysphoric disorder are experienced by 3–8% of women. The menstrual cycle can be modified by hormonal nativity control.
Cycles and phases [edit]
Progression of the menstrual cycle and some of the hormones contributing to it
The menstrual bicycle encompasses the ovarian and uterine cycles. The ovarian cycle describes changes that occur in the follicles of the ovary,[1] whereas the uterine bicycle describes changes in the endometrial lining of the uterus. Both cycles tin can exist divided into phases. The ovarian cycle consists of alternate follicular and luteal phases, and the uterine cycle consists of catamenia, the proliferative stage, and the secretory phase.[2] The menstrual bicycle is controlled past the hypothalamus and the pituitary gland in the brain. The hypothalamus releases gonadotropin-releasing hormone (GnRH), which causes the nearby inductive pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Before puberty, GnRH is released in low steady quantities and at a steady rate. After puberty, GnRH is released in large pulses, and the frequency and magnitude of these determine how much FSH and LH are produced past the pituitary.[3]
Measured from the first twenty-four hours of i period to the first mean solar day of the next, the length of a menstrual cycle varies but has a median length of 28 days.[iv] The cycle is often less regular at the beginning and end of a woman's reproductive life.[4] At puberty, a child's trunk begins to mature into an adult body capable of sexual reproduction; the first period (chosen menarche) occurs at effectually 12 years of age and continues for about 30–45 years.[5] [6] Menstrual cycles end at menopause, which is usually between 45 and 55 years of age.[7] [8]
Ovarian wheel [edit]
Between menarche and menopause the homo ovaries regularly alternate betwixt luteal and follicular phases during the monthly menstrual wheel.[nine] Stimulated by gradually increasing amounts of estrogen in the follicular phase, discharges of blood period finish and the uterine lining thickens. Follicles in the ovary brainstorm developing under the influence of a complex coaction of hormones, and afterward several days one, or occasionally two, become dominant, while non-dominant follicles shrink and die. About mid-cycle, some 10–12 hours after the luteinizing hormone (LH) surges,[4] the dominant follicle releases an oocyte, in an upshot called ovulation.[10]
After ovulation, the oocyte lives for 24 hours or less without fertilization,[11] while the remains of the dominant follicle in the ovary become a corpus luteum – a body with the master function of producing large amounts of the hormone progesterone.[12] [a] Nether the influence of progesterone, the uterine lining changes to prepare for potential implantation of an embryo to found a pregnancy. The thickness of the endometrium continues to increase in response to mounting levels of estrogen, which is released by the antral follicle (a mature ovarian follicle) into the claret circulation. Acme levels of estrogen are reached at around 24-hour interval thirteen of the cycle and coincide with ovulation. If implantation does not occur within about ii weeks, the corpus luteum degenerates into the corpus albicans, which does not produce hormones, causing a sharp drop in levels of both progesterone and estrogen. This drop causes the uterus to lose its lining in menstruation; it is effectually this time that the lowest levels of estrogen are reached.[14]
In an ovulatory menstrual bicycle, the ovarian and uterine cycles are concurrent and coordinated and terminal between 21 and 35 days in an adult adult female, with a population average of 27–29 days.[xv] Although the average length of the homo menstrual bike is similar to that of the lunar cycle, there is no causal relation between the two.[sixteen]
Follicular phase [edit]
The ovaries contain a finite number of egg stem cells, granulosa cells and theca cells, which together course primordial follicles.[12] At effectually twenty weeks into gestation some 7 million immature eggs have already formed in an ovary. This decreases to effectually 2 1000000 by the time a girl is born, and 300,000 by the time she has her offset period. On boilerplate, one egg matures and is released during ovulation each month subsequently menarche.[17] Beginning at puberty, these mature to main follicles independently of the menstrual cycle.[18] The development of the egg is called oogenesis and merely one cell survives the divisions to look fertilization. The other cells are discarded equally polar bodies, which cannot be fertilized.[19] The follicular phase is the commencement part of the ovarian bicycle and it ends with the completion of the antral follicles.[9] Meiosis (cell sectionalization) remains incomplete in the egg cells until the antral follicle is formed. During this stage usually only one ovarian follicle fully matures and gets ready to release an egg.[20] The follicular stage shortens significantly with age, lasting around 14 days in women aged 18–24 compared with 10 days in women aged 40–44.[14]
Through the influence of a rise in follicle stimulating hormone (FSH) during the first days of the bicycle, a few ovarian follicles are stimulated. These follicles, which have been developing for the better role of a year in a process known as folliculogenesis, compete with each other for authorisation. All but 1 of these follicles will stop growing, while one dominant follicle – the one that has the about FSH receptors – will continue to maturity. The remaining follicles dice in a process called follicular atresia.[21] Luteinizing hormone (LH) stimulates further development of the ovarian follicle. The follicle that reaches maturity is chosen an antral follicle, and it contains the ovum (egg cell).[22]
The theca cells develop receptors that bind LH, and in response secrete large amounts of androstenedione. At the same time the granulosa cells surrounding the maturing follicle develop receptors that bind FSH, and in response start secreting androstenedione, which is converted to estrogen by the enzyme aromatase. The estrogen inhibits farther production of FSH and LH by the pituitary gland. This negative feedback regulates levels of FSH and LH. The dominant follicle continues to secrete estrogen, and the rise estrogen levels make the pituitary more responsive to GnRH from the hypothalamus. As estrogen increases this becomes a positive feedback signal, which makes the pituitary secrete more FSH and LH. This surge of FSH and LH usually occurs 1 to two days earlier ovulation and is responsible for stimulating the rupture of the antral follicle and release of the oocyte.[18] [23]
Ovulation [edit]

An ovary nearly to release an egg
Around twenty-four hour period fourteen, the egg is released from the ovary.[24] Called "ovulation", this occurs when a mature egg is released from the ovarian follicles into the fallopian tube, about 10–12 hours afterwards the height in LH surge.[4] Typically simply 1 of the 15–20 stimulated follicles reaches total maturity, and simply ane egg is released.[25] Ovulation just occurs in effectually ten% of cycles during the start two years following menarche, and past the age of xl–l, the number of ovarian follicles is depleted.[26] LH initiates ovulation at effectually day fourteen and stimulates the formation of the corpus luteum.[2] Following further stimulation by LH, the corpus luteum produces and releases estrogen, progesterone, relaxin (which relaxes the uterus by inhibiting contractions of the myometrium), and inhibin (which inhibits farther secretion of LH).[22]
The release of LH matures the egg and weakens the follicle wall in the ovary, causing the fully developed follicle to release its oocyte.[27] If information technology is fertilized past a sperm, the oocyte promptly matures into an ootid, which blocks the other sperm cells and becomes a mature egg. If information technology is not fertilized by a sperm, the oocyte degenerates. The mature egg has a diameter of about 0.1 mm (0.0039 in),[28] and is the largest man cell.[29]
Which of the two ovaries – left or right – ovulates appears random;[xxx] no left and correct coordinating procedure is known.[31] Occasionally both ovaries release an egg; if both eggs are fertilized, the outcome is fraternal twins.[32] After release from the ovary, the egg is swept into the fallopian tube past the fimbria – a fringe of tissue at the terminate of each fallopian tube. After about a day, an unfertilized egg disintegrates or dissolves in the fallopian tube, and a fertilized egg reaches the uterus in iii to 5 days.[33]
Fertilization usually takes place in the ampulla, the widest section of the fallopian tubes. A fertilized egg immediately starts the process of embryogenesis (development). The developing embryo takes near three days to reach the uterus, and another three days to implant into the endometrium. It has normally reached the blastocyst stage at the time of implantation: this is when pregnancy begins.[34] The loss of the corpus luteum is prevented by fertilization of the egg. The syncytiotrophoblast (the outer layer of the resulting embryo-containing blastocyst that later becomes the outer layer of the placenta) produces man chorionic gonadotropin (hCG), which is very similar to LH and preserves the corpus luteum. During the starting time few months of pregnancy, the corpus luteum continues to secrete progesterone and estrogens at slightly college levels than those at ovulation. Afterward this and for the rest of the pregnancy, the placenta secretes high levels of these hormones – along with homo chorionic gonadotropin (hCG), which stimulates the corpus luteum to secrete more progesterone and estrogens, blocking the menstrual cycle.[35] These hormones as well ready the mammary glands for milk[b] product.[35]
Luteal phase [edit]
Lasting about 14 days,[4] the luteal phase is the final phase of the ovarian cycle and it corresponds to the secretory stage of the uterine bicycle. During the luteal phase, the pituitary hormones FSH and LH cause the remaining parts of the ascendant follicle to transform into the corpus luteum, which produces progesterone.[37] [c] The increased progesterone starts to induce the production of estrogen. The hormones produced by the corpus luteum also suppress production of the FSH and LH that the corpus luteum needs to maintain itself. The level of FSH and LH fall quickly, and the corpus luteum atrophies.[39] Falling levels of progesterone trigger menstruation and the beginning of the side by side cycle. From the time of ovulation until progesterone withdrawal has acquired menstruum to brainstorm, the process typically takes about 2 weeks. For an individual woman, the follicular stage often varies in length from cycle to wheel; by contrast, the length of her luteal phase will exist fairly consequent from cycle to wheel at 10 to 16 days (average 14 days).[xiv]
Uterine bicycle [edit]

The beefcake of the uterus
The uterine bicycle has three phases: menses, proliferative and secretory.[40]
Catamenia [edit]
Menses (too chosen menstrual bleeding, menses or a period) is the offset and most evident phase of the uterine cycle and get-go occurs at puberty. Called menarche, the starting time flow occurs at the age of around twelve or thirteen years.[eight] The average age is by and large after in the developing world and earlier in developed world.[41] In precocious puberty, it can occur every bit early as historic period eight years,[42] and this tin still exist normal.[43] [44]
Menstruation is initiated each month by falling levels of estrogen and progesterone and the release of prostaglandins,[20] which constrict the spiral arteries. This causes them to spasm, contract and break up.[45] The blood supply to the endometrium is cutting off and the cells of the acme layer of the endometrium (the stratum functionalis) get deprived of oxygen and die. Later the whole layer is lost and only the bottom layer, the stratum basalis, is left in identify.[twenty] An enzyme chosen plasmin breaks up the blood clots in the menstrual fluid, which eases the menstruation of blood and cleaved downwards lining from the uterus.[46] The flow of claret continues for two–6 days and effectually 30–sixty milliliters of claret is lost,[15] and is a sign that pregnancy has not occurred.[47]
The flow of blood normally serves as a sign that a woman has non get pregnant, but this cannot be taken every bit certainty, as several factors can cause bleeding during pregnancy.[48] Menstruum occurs on boilerplate one time a month from menarche to menopause, which corresponds with a woman'south fertile years. The average age of menopause in women is 52 years, and it typically occurs between 45 and 55 years of age.[49] Menopause is preceded by a stage of hormonal changes chosen perimenopause.[7]
Eumenorrhea denotes normal, regular menstruation that lasts for around the first 5 days of the cycle.[24] Women who experience menorrhagia (heavy menstrual bleeding) are more than susceptible to atomic number 26 deficiency than the boilerplate person.[50]
Proliferative stage [edit]

During the menstrual cycle, levels of estradiol (an estrogen) vary past 200 percent. Levels of progesterone vary by over 1200 percent.[51]
The proliferative phase is the second stage of the uterine wheel when estrogen causes the lining of the uterus to grow and proliferate.[39] The latter part of the follicular phase overlaps with the proliferative phase of the uterine cycle.[30] Equally they mature, the ovarian follicles secrete increasing amounts of estradiol, an estrogen. The estrogens initiate the formation of a new layer of endometrium in the uterus with the spiral arterioles.[2]
As estrogen levels increase, cells in the cervix produce a type of cervical mucus[52] that has a higher pH and is less viscous than usual, rendering it more friendly to sperm.[53] This increases the chances of fertilization, which occurs around day 11 to solar day xiv.[11] This cervical mucus can be detected equally a vaginal discharge that is copious and resembles raw egg whites.[54] For women who are practicing fertility sensation, information technology is a sign that ovulation may be about to take identify,[54] but it does not mean ovulation will definitely occur.[fifteen]
Secretory phase [edit]
The secretory phase is the concluding phase of the uterine cycle and information technology corresponds to the luteal phase of the ovarian cycle. During the secretory stage, the corpus luteum produces progesterone, which plays a vital part in making the endometrium receptive to the implantation of a blastocyst (a fertilized egg, which has begun to abound).[55] Glycogen, lipids, and proteins are secreted into the uterus[56] and the cervical mucus thickens.[57] In early pregnancy progesterone also increases blood catamenia and reduces the contractility of the polish musculus in the uterus[22] and raises the woman'south basal body temperature.[58]
If pregnancy does non occur the ovarian and uterine cycles start over again.[46]
Anovulatory cycles and short luteal phases [edit]
But ii thirds of overtly normal menstrual cycles are ovulatory, that is, cycles in which ovulation occurs.[xv] The other third lack ovulation or have a curt luteal phase (less than x days[59]) in which progesterone production is insufficient for normal physiology and fertility.[threescore] Cycles in which ovulation does not occur (anovulation) are common in girls who accept just begun menstruating and in women around menopause. During the first two years post-obit menarche, ovulation is absent in around half of cycles. V years after menarche, ovulation occurs in around 75% of cycles and this reaches 80% in the following years.[61] Anovulatory cycles are oft overtly identical to normally ovulatory cycles.[62] Whatsoever alteration to residue of hormones tin can lead to anovulation. Stress, feet and eating disorders tin can cause a fall in GnRH, and a disruption of the menstrual bicycle. Chronic anovulation occurs in vi–15% of women during their reproductive years. Around menopause, hormone feedback dysregulation leads to anovulatory cycles. Although anovulation is not considered a affliction, it tin can be a sign of an underlying condition such equally polycystic ovary syndrome.[63] Anovulatory cycles or short luteal phases are normal when women are nether stress or athletes increasing the intensity of training. These changes are reversible as the stressors decrease or, in the example of the athlete, equally she adapts to the training.[59]
Menstrual health [edit]

A man chief ovarian follicle viewed by microscopy. The round oocyte stained red in the center is surrounded by a layer of granulosa cells, which are enveloped by the basement membrane and theca cells. The magnification is around 1000 times. (H&E stain)
Although a normal and natural process,[64] some women feel problems sufficient to disrupt their lives as a result of their menstrual bicycle.[65] These include acne, tender breasts, feeling tired, and premenstrual syndrome (PMS).[65] [66] More astringent problems such equally premenstrual dysphoric disorder are experienced by three to 8% of women.[4] [67] Dysmenorrhea or "period pain"[68] tin can crusade cramps in the abdomen, back, or upper thighs that occur during the first few days of flow.[69] Debilitating period pain is not normal and can be a sign of something astringent such every bit endometriosis.[70] These bug can significantly affect a woman's health and quality of life and timely interventions can improve the lives of these women.[71]
There are common culturally communicated misbeliefs that the menstrual cycle affects women's moods, causes depression or irritability, or that menstruation is a painful, shameful or unclean feel. Ofttimes a woman's normal mood variation is falsely attributed to the menstrual bicycle. Much of the research is weak, but at that place appears to be a very pocket-sized increase in mood fluctuations during the luteal and menstrual phases, and a respective decrease during the residue of the bike.[72] Changing levels of estrogen and progesterone across the menstrual cycle exert systemic furnishings on aspects of physiology including the brain, metabolism, and musculoskeletal arrangement. The issue tin be subtle physiological and observable changes to women'due south athletic performance including strength, aerobic, and anaerobic performance.[73] Changes to the brain accept also been observed throughout the menstrual cycle[74] but exercise not interpret into measurable changes in intellectual achievement – including bookish functioning, trouble-solving, memory, and creativity.[75] Improvements in spatial reasoning ability during the menstruation phase of the cycle are probably caused past decreases in levels of estrogen and progesterone.[72]
In some women, ovulation features a feature pain[d] called mittelschmerz (a High german term meaning middle hurting). The cause of the hurting is associated with the ruptured follicle, causing a pocket-size amount of blood loss.[twenty]
Even when normal, the changes in hormone levels during the menstrual cycle can increase the incidence of disorders such as autoimmune diseases,[79] which might be caused by estrogen enhancement of the allowed organisation.[four]
Around 40% of women with epilepsy find that their seizures occur more than frequently at certain phases of their menstrual cycle. This catamenial epilepsy may be due to a drib in progesterone if it occurs during the luteal stage or effectually menstruation, or a surge in estrogen if information technology occurs at ovulation. Women who have regular periods tin can take medication simply before and during period. Options include progesterone supplements, increasing the dose of their regular anticonvulsant drug, or temporarily adding an anticonvulsant such as clobazam or acetazolamide. If this is ineffective, or when a adult female's menstrual wheel is irregular, and then handling is to cease the menstrual cycle occurring. This may be achieved using medroxyprogesterone, triptorelin or goserelin, or past sustained use of oral contraceptives.[80] [81]
Hormonal contraception [edit]
Hormonal contraceptives prevent pregnancy past inhibiting the secretion of the hormones, FSH, LH and GnRH. Hormonal contraception that contains estrogen, such every bit combined oral contraceptive pills (COCs, often referred to as birth command pills) finish the development of the dominant follicle and the mid-wheel LH surge and thus ovulation.[82] Sequential dosing and discontinuation of the COC can mimic the uterine cycle and produce bleeding that resembles a flow. In some cases, this bleeding is lighter.[83]
Progestin-only methods of hormonal contraception do not always prevent ovulation just instead piece of work past stopping the cervical fungus from becoming sperm-friendly. Hormonal contraception is available in a variety of forms such every bit pills, patches, peel implants and hormonal intrauterine devices (IUDs).[84]
Evolution and other species [edit]
Most female person mammals take an estrous cycle, merely only ten primate species, 4 bat species, the elephant shrews and the spiny mouse species Cairo spiny mouse (Acomys cahirinus) have a menstrual cycle.[85] [86] The cycles are the same equally in humans autonomously from the length, which ranges from 9 to 37 days.[87] [85] The lack of immediate relationship between these groups suggests that 4 distinct evolutionary events take caused menstruation to arise.[88] In species that accept a menstrual cycle, ovulation is non obvious to potential mates and there is no mating season.[89] [ninety] There are four theories on the evolutionary significance of menses:[88]
- Control of sperm-borne pathogens.[91] [92] [93] This hypothesis held that flow protected the uterus against pathogens introduced past sperm. Hypothesis 1 does not accept into business relationship that copulation can take place weeks before menstruation and that potentially infectious semen is not controlled by menstruum in other species.[88]
- Free energy conservation.[92] [94] This hypothesis claimed that it took less free energy to rebuild a uterine lining than to maintain it if pregnancy did not occur. Hypothesis two does non explain other species that besides exercise not maintain a uterine lining but do not menstruate.[88]
- A theory based on spontaneous decidualization (a procedure that results in significant changes to cells of the endometrium in preparation for, and during, pregnancy, in which the endometrium changes into the decidua). Decidualization leads to the development of the endothelium, which involves cells of the allowed organisation,[87] the germination of a new claret supply, hormones and tissue differentiation. In non-menstruating mammals, decidualization is driven by the embryo, not the mother.[92] It evolved in some placental mammals considering it confers advantages in that it allows females to fix for pregnancy without needing a signal from the fetus.[88] Hypothesis three defers to an explanation of the evolutionary origin of spontaneous decidualization and does not explicate the evolution of menses lone.[88]
- Uterine pre-conditioning.[95] This hypothesis claims that a monthly pre-conditioning of the uterus is needed in species, such as humans, that have deeply invasive (deep-rooted) placentas. In the procedure leading to the formation of a placenta, maternal tissues are invaded. This hypothesis holds that menstruation was not evolutionary, rather the result of a casual pre-conditioning of the uterus to protect uterine tissue from the deeply rooting placenta, in which a thicker endometrium develops.[95] Hypothesis iv does not explain menstruation in non-primates.[88]
Notes [edit]
- ^ Progesterone levels exceed those of estrogen (estradiol) by a hundred-fold.[13]
- ^ Breastfeeding women tin experience complete suppression of follicular development, follicular development but no ovulation, or resumption of normal menstrual cycles.[36]
- ^ In the corpus luteum, cholesterol side-chain cleavage enzyme converts cholesterol to pregnenolone, which is converted to progesterone.[38]
- ^ Uncharacteristic mid-cycle pain may exist caused by medical conditions such as ectopic pregnancy or ruptured ovarian cyst[76] [77] or may be confused with appendicitis.[78]
References [edit]
- ^ Richards JS (2018). "The ovarian cycle". Vitamins and Hormones (Review). 107: 1–25. doi:10.1016/bs.vh.2018.01.009. ISBN978-0-128-14359-9. PMID 29544627.
- ^ a b c Tortora 2017, p. 944.
- ^ Prior 2020, p. 42.
- ^ a b c d e f g Reed BF, Carr BR, Feingold KR, et al. (2018). "The Normal Menstrual Bicycle and the Control of Ovulation". Endotext (Review). PMID 25905282. Archived from the original on 28 May 2021. Retrieved eight January 2021.
- ^ Prior 2020, p. xl.
- ^ Lacroix AE, Gondal H, Langaker MD (2020). "Physiology, menarche". StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (Review). PMID 29261991.
- ^ a b Rodriguez-Landa 2017, p. 8.
- ^ a b Papadimitriou A (Dec 2016). "The evolution of the age at menarche from prehistorical to modern times". Journal of Pediatric and Boyish Gynecology (Review). 29 (six): 527–30. doi:10.1016/j.jpag.2015.12.002. PMID 26703478.
- ^ a b Sherwood 2016, p. 741.
- ^ Sherwood 2016, p. 747.
- ^ a b Tortora 2017, p. 957.
- ^ a b Tortora 2017, p. 929.
- ^ Prior 2020, p. 41.
- ^ a b c Tortora 2017, pp. 942–46.
- ^ a b c d Prior 2020, p. 45.
- ^ Norris & Carr 2013, p. 361.
- ^ Ugwumadu 2014, p. 115.
- ^ a b Watchman 2020, p. 8.
- ^ Schmerler S, Wessel GM (January 2011). "Polar bodies – more than a lack of understanding than a lack of respect". Molecular Reproduction and Evolution (Review). 78 (1): 3–eight. doi:10.1002/mrd.21266. PMC3164815. PMID 21268179.
- ^ a b c d Tortora 2017, p. 945.
- ^ Johnson 2007, p. 86.
- ^ a b c Tortora 2017, p. 942.
- ^ Sherwood 2016, p. 745.
- ^ a b Tortora 2017, p. 943.
- ^ Sadler 2019, p. 48.
- ^ Tortora 2017, p. 953.
- ^ Sherwood 2016, p. 746.
- ^ Alberts B, Johnson A, Lewis J, Raff Yard, Roberts Thou, Walter P (2002). "Eggs". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN0-8153-3218-1. Archived from the original on 16 December 2019. Retrieved 25 Feb 2021.
- ^ Iussig B, Maggiulli R, Fabozzi G, Bertelle South, Vaiarelli A, Cimadomo D, Ubaldi FM, Rienzi L (May 2019). "A brief history of oocyte cryopreservation: Arguments and facts". Acta Obstetricia et Gynecologica Scandinavica (Review). 98 (v): 550–58. doi:10.1111/aogs.13569. PMID 30739329.
- ^ a b Parker 2019, p. 283.
- ^ Johnson 2007, pp. 192–93.
- ^ Johnson 2007, p. 192.
- ^ Sadler 2019, p. 36.
- ^ Tortora 2017, p. 959.
- ^ a b Tortora 2017, p. 976.
- ^ Carr SL, Gaffield ME, Dragoman MV, Phillips S (September 2016). "Safety of the progesterone-releasing vaginal ring (PVR) among lactating women: A systematic review". Contraception (Review). 94 (3): 253–61. doi:ten.1016/j.contraception.2015.04.001. PMID 25869631.
- ^ Johnson 2007, p. 91.
- ^ King SR, LaVoie HA (January 2012). "Gonadal transactivation of STARD1, CYP11A1 and HSD3B". Frontiers in Bioscience (Landmark Edition). 17: 824–46. doi:10.2741/3959. PMID 22201776.
- ^ a b Ugwumadu 2014, p. 117.
- ^ Salamonsen LA (December 2019). "Women in reproductive scientific discipline: Agreement human endometrial function". Reproduction (Cambridge, England) (Review). 158 (vi): F55–F67. doi:10.1530/REP-eighteen-0518. PMID 30521482.
- ^ Alvergne A, Högqvist Tabor V (June 2018). "Is female health cyclical? Evolutionary perspectives on menstruation". Trends in Ecology & Evolution (Review). 33 (6): 399–414. arXiv:1704.08590. doi:10.1016/j.tree.2018.03.006. PMID 29778270. S2CID 4581833.
- ^ Ibitoye M, Choi C, Tai H, Lee G, Sommer M (2017). "Early menarche: A systematic review of its effect on sexual and reproductive health in low- and heart-income countries". PLOS One (Review). 12 (6): e0178884. Bibcode:2017PLoSO..1278884I. doi:10.1371/journal.pone.0178884. PMC5462398. PMID 28591132.
- ^ "Menstruation and the menstrual bicycle fact sheet". Office of Women'southward Health. US Department of Health and Human being Services. 23 Dec 2014. Archived from the original on 26 June 2015. Retrieved 25 June 2015.
- ^ Sultan C, Gaspari L, Maimoun L, Kalfa North, Paris F (April 2018). "Disorders of puberty" (PDF). Best Practice & Research. Clinical Obstetrics & Gynaecology (Review). 48: 62–89. doi:10.1016/j.bpobgyn.2017.11.004. PMID 29422239. Archived (PDF) from the original on 1 July 2020. Retrieved 27 February 2021.
- ^ Johnson 2007, p. 152.
- ^ a b Tortora 2017, p. 600.
- ^ Johnson 2007, p. 99.
- ^ Cakewalk C (May 2016). "Early on pregnancy haemorrhage". Australian Family Doctor (Review). 45 (5): 283–86. PMID 27166462.
- ^ Towner MC, Nenko I, Walton SE (April 2016). "Why do women finish reproducing before menopause? A life-history arroyo to age at last birth". Philosophical Transactions of the Regal Society of London. Serial B, Biological Sciences (Review). 371 (1692): 20150147. doi:10.1098/rstb.2015.0147. PMC4822427. PMID 27022074.
- ^ Harvey LJ, Armah CN, Squeamish JR, Foxall RJ, John Lewis D, Langford NJ, Fairweather-Tait SJ (October 2005). "Bear upon of menstrual blood loss and nutrition on iron deficiency amongst women in the UK". The British Journal of Nutrition (Comparative report). 94 (4): 557–64. doi:10.1079/BJN20051493. PMID 16197581.
- ^ Prior JC (2020). "Women'southward reproductive organization every bit balanced estradiol and progesterone actions—A revolutionary, paradigm-shifting concept in women'southward health". Drug Discovery Today: Illness Models. 32, Office B: 31–40. doi:10.1016/j.ddmod.2020.11.005.
- ^ Simmons RG, Jennings 5 (July 2020). "Fertility sensation-based methods of family unit planning". Best Practice & Research. Clinical Obstetrics & Gynaecology (Review). 66: 68–82. doi:10.1016/j.bpobgyn.2019.12.003. PMID 32169418.
- ^ Tortora 2017, pp. 936–37.
- ^ a b Su HW, Yi YC, Wei TY, Chang TC, Cheng CM (September 2017). "Detection of ovulation, a review of currently available methods". Bioeng Transl Med (Review). 2 (iii): 238–46. doi:10.1002/btm2.10058. PMC5689497. PMID 29313033.
- ^ Lessey BA, Immature SL (April 2019). "What exactly is endometrial receptivity?". Fertility and Sterility (Review). 111 (4): 611–17. doi:10.1016/j.fertnstert.2019.02.009. PMID 30929718.
- ^ Salamonsen LA, Evans J, Nguyen HP, Edgell TA (March 2016). "The microenvironment of human implantation: determinant of reproductive success". American Periodical of Reproductive Immunology (Review). 75 (iii): 218–25. doi:10.1111/aji.12450. PMID 26661899.
- ^ Han Fifty, Taub R, Jensen JT (Nov 2017). "Cervical mucus and contraception: what we know and what we don't". Contraception (Review). 96 (5): 310–321. doi:x.1016/j.contraception.2017.07.168. PMID 28801053.
- ^ Charkoudian Northward, Hart EC, Barnes JN, Joyner MJ (June 2017). "Autonomic control of torso temperature and blood pressure: influences of female sex hormones" (PDF). Clinical Autonomic Inquiry (Review). 27 (3): 149–55. doi:x.1007/s10286-017-0420-z. hdl:1983/c0c1058c-553b-4563-8dd1-b047d9b672c1. PMID 28488202. S2CID 3773043. Archived (PDF) from the original on 10 May 2020. Retrieved 27 Feb 2021.
- ^ a b Liu AY, Petit MA, Prior JC (2020). "Do and the hypothalamus: ovulatory adaptations". In Hackney Air-conditioning, Constantini NW (eds.). Endocrinology of Physical Activeness and Sport. Contemporary Endocrinology. Springer International Publishing. pp. 124–47. doi:10.1007/978-iii-030-33376-8_8. ISBN978-3-030-33376-8. S2CID 243129220.
- ^ Prior 2020, p. 46.
- ^ Elmaoğulları S, Aycan Z (July 2018). "Abnormal uterine bleeding in adolescents". Journal of Clinical Research in Pediatric Endocrinology. ten (iii): 191–97. doi:10.4274/jcrpe.0014. PMC6083466. PMID 29537383.
- ^ Prior 2020, p. 44.
- ^ Hernandez-Rey, AE (2 Baronial 2018). "Anovulation". Medscape. Medscape LLC. Archived from the original on 20 March 2021. Retrieved xxx March 2021.
- ^ Prior 2020, p. 50.
- ^ a b Gudipally PR, Sharma GK (2020). "Premenstrual syndrome". StatPearls [Internet] (Review). PMID 32809533.
- ^ Ferries-Rowe E, Corey E, Archer JS (November 2020). "Primary Dysmenorrhea: Diagnosis and Therapy". Obstetrics and Gynecology. 136 (5): 1047–1058. doi:x.1097/AOG.0000000000004096. PMID 33030880.
- ^ Appleton SM (March 2018). "Premenstrual syndrome: evidence-based evaluation and treatment". Clinical Obstetrics and Gynecology (Review). 61 (i): 52–61. doi:x.1097/GRF.0000000000000339. PMID 29298169. S2CID 28184066.
- ^ Nagy H, Khan MA (2020). "Dysmenorrhea". StatPearls (Review). PMID 32809669.
- ^ Bakery FC, Lee KA (September 2018). "Menstrual cycle effects on slumber". Slumber Medicine Clinics (Review). 13 (3): 283–94. doi:10.1016/j.jsmc.2018.04.002. PMID 30098748. S2CID 51968811.
- ^ Maddern J, Grundy L, Castro J, Brierley SM (2020). "Hurting in endometriosis". Frontiers in Cellular Neuroscience. fourteen: 590823. doi:10.3389/fncel.2020.590823. PMC7573391. PMID 33132854.
- ^ Matteson KA, Zaluski KM (September 2019). "Menstrual health as a part of preventive health care". Obstetrics and Gynecology Clinics of North America (Review). 46 (3): 441–53. doi:10.1016/j.ogc.2019.04.004. PMID 31378287. S2CID 199437314.
- ^ a b Else-Quest & Hyde 2021, pp. 258–61.
- ^ Carmichael MA, Thomson RL, Moran LJ, Wycherley TP (Feb 2021). "The bear upon of menstrual wheel phase on athletes' performance: a narrative review". Int J Environ Res Public Health (Review). xviii (iv): 1667. doi:ten.3390/ijerph18041667. PMC7916245. PMID 33572406.
- ^ Pletzer B, Harris TA, Scheuringer A, Hidalgo-Lopez E (October 2019). "The cycling brain: menstrual cycle related fluctuations in hippocampal and fronto-striatal activation and connectivity during cognitive tasks". Neuropsychopharmacology. 44 (xi): 1867–75. doi:10.1038/s41386-019-0435-three. PMC6785086. PMID 31195407.
- ^ Le J, Thomas Due north, Gurvich C (March 2020). "Cognition, the menstrual bicycle, and premenstrual disorders: a review". Brain Sci (Review). ten (four): 198. doi:ten.3390/brainsci10040198. PMC7226433. PMID 32230889.
- ^ Kruszka PS, Kruszka SJ (July 2010). "Evaluation of acute pelvic pain in women". Am Fam Medico (Review). 82 (ii): 141–47. PMID 20642266. Archived from the original on 27 Jan 2021. Retrieved 4 March 2021.
- ^ Cleary Thou, Flanagan KW (2019). Acute and Emergency Care in Athletic Training. Homo Kinetics. p. 340.
- ^ Brott NR, Le JK (2020). "Mittelschmerz". Stat Pearls [Internet] (Review). PMID 31747229. Archived from the original on 28 May 2021. Retrieved 4 March 2021.
- ^ Talsania M, Scofield RH (May 2017). "Menopause and rheumatic disease". Rheumatic Disease Clinics of North America (Review). 43 (2): 287–302. doi:10.1016/j.rdc.2016.12.011. PMC5385852. PMID 28390570.
- ^ Maguire MJ, Nevitt SJ (September 2021). "Treatments for seizures in catamenial (menstrual-related) epilepsy". The Cochrane Database of Systematic Reviews. 2021 (9): CD013225. doi:10.1002/14651858.CD013225.pub3. PMC 8444032. PMID 34528245.
- ^ Sveinsson O, Tomson T (September 2014). "Epilepsy and menopause: potential implications for pharmacotherapy". Drugs & Aging. 31 (9): 671–75. doi:10.1007/s40266-014-0201-5. PMID 25079452. S2CID 21166687.
- ^ Tortora 2017, p. 948.
- ^ Polis CB, Hussain R, Drupe A (June 2018). "There might be blood: a scoping review on women'southward responses to contraceptive-induced menstrual bleeding changes". Reproductive Health. 15 (1): 114. doi:ten.1186/s12978-018-0561-0. PMC6020216. PMID 29940996.
- ^ Tortora 2017, pp. 948–49.
- ^ a b Bellofiore Due north, Ellery SJ, Mamrot J, Walker DW, Temple-Smith P, Dickinson H (Jan 2017). "Showtime bear witness of a menstruating rodent: the spiny mouse (Acomys cahirinus)" (PDF). American Journal of Obstetrics and Gynecology (Periodical commodity). 216 (i): 40.e1–40.e11. doi:10.1016/j.ajog.2016.07.041. PMID 27503621. S2CID 88779.
- ^ Bellofiore, Nadia; Cousins, Fiona; Temple-Smith, Peter; Evans, Jemma (ane Feb 2019). "Altered exploratory behaviour and increased food intake in the spiny mouse earlier menstruation: a unique pre-clinical model for examining premenstrual syndrome". Homo Reproduction. 34 (2): 308–322. doi:ten.1093/humrep/dey360. ISSN 0268-1161. PMID 30561655.
- ^ a b Catalini L, Fedder J (May 2020). "Characteristics of the endometrium in menstruating species: lessons learned from the animal kingdom†". Biology of Reproduction (Journal article). 102 (six): 1160–69. doi:ten.1093/biolre/ioaa029. PMC7253787. PMID 32129461.
- ^ a b c d e f g Emera D, Romero R, Wagner G (Jan 2012). "The development of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness". BioEssays (Periodical article). 34 (ane): 26–35. doi:10.1002/bies.201100099. PMC3528014. PMID 22057551. Meet BBC Earth lay summary, 20 Apr 2015.
- ^ Schjenken JE, Robertson SA (July 2020). "The female person response to seminal fluid". Physiological Reviews (Review). 100 (3): 1077–117. doi:10.1152/physrev.00013.2018. PMID 31999507. S2CID 210983017.
- ^ Muller MN (May 2017). "Testosterone and reproductive effort in male primates". Hormones and Behavior (Review). 91: 36–51. doi:x.1016/j.yhbeh.2016.09.001. PMC5342957. PMID 27616559.
- ^ Martin RD (2007). "The development of homo reproduction: a primatological perspective". American Periodical of Concrete Anthropology (Review). 134 (S45): 59–84. doi:10.1002/ajpa.20734. PMID 18046752.
- ^ a b c Finn CA (June 1998). "Menstruation: a nonadaptive issue of uterine evolution". The Quarterly Review of Biological science (Review). 73 (2): 163–73. doi:10.1086/420183. PMID 9618925. S2CID 25135630.
- ^ Profet M (September 1993). "Menstruation equally a defence against pathogens transported past sperm". The Quarterly Review of Biology (Review). 68 (three): 335–86. doi:10.1086/418170. PMID 8210311. S2CID 23738569.
- ^ Strassmann BI (June 1996). "The evolution of endometrial cycles and menstruum". The Quarterly Review of Biology (Review). 71 (2): 181–220. doi:x.1086/419369. PMID 8693059. S2CID 6207295.
- ^ a b Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA (June 2009). "A part for catamenia in preconditioning the uterus for successful pregnancy". American Periodical of Obstetrics and Gynecology (Periodical commodity). 200 (6): 615.e1–half dozen. doi:10.1016/j.ajog.2008.11.037. PMID 19136085.
Book sources [edit]
- Else-Quest Due north, Hyde JS (2021). "Psychology, gender, and wellness: psychological aspects of the menstrual bike". The Psychology of Women and Gender: Half the Human Experience + (10th ed.). Los Angeles: SAGE Publishing. ISBN978-1-544-39360-5.
- Johnson MH (2007). Essential Reproduction. Malden, Massachusetts: Wiley-Blackwell. ISBN978-1-4051-1866-8. OCLC 76074156.
- Norris DA, Carr JA (2013). Vertebrate Endocrinology (fifth ed.). Academic Press. ISBN978-0-123-96465-6.
- Parker S (2019). The Concise Human Body Book: An Illustrated Guide to its Structures, Function and Disorders. London: DK. ISBN978-0-241-39552-3. OCLC 1091644711.
- Prior JC (2020). "The menstrual cycle: its biological science in the context of silent ovulatory disturbances". In Ussher JM, Chrisler JC, Perz J (eds.). Routledge International Handbook of Women's Sexual and Reproductive Health (1st ed.). Abingdon, Oxon: Routledge. ISBN978-1-138-49026-0. OCLC 1121130010.
- Rodriguez-Landa J (2017). A Multidisciplinary Look at Menopause. Rijeka, Croatia: IntechOpen. ISBN978-953-51-3405-3. OCLC 1193045564.
- Sadler TW (2019). Langman's Medical Embryology. Philadelphia: Wolters Kluwer. ISBN978-i-4963-8390-seven. OCLC 1042400100.
- Sherwood Fifty (2016). Human Physiology: From Cells to Systems. Boston, Massachusetts: Cengage Learning. ISBN978-1-285-86693-2. OCLC 905848832.
- Tortora G (2017). Tortora's Principles of Beefcake & Physiology. Hoboken, New Jersey: John Wiley & Sons, Inc. ISBN978-1-119-38292-8. OCLC 990424568.
- Ugwumadu A (2014). Basic Sciences for Obstetrics and Gynaecology: Cadre Material for MRCOG. Oxford, England: Oxford University Press. ISBN978-0-xix-953508-viii. OCLC 889303297.
- Watchman T (2020). Zero to Finals : Obstetrics and Gynaecology. Manchester: Zero to Finals. ISBN979-8-6037-9726-vii. OCLC 1233034578.
External links [edit]
![]() Media related to Menstrual wheel at Wikimedia Commons
Media related to Menstrual wheel at Wikimedia Commons
Source: https://en.wikipedia.org/wiki/Menstrual_cycle
Posted by: laforestoulds1946.blogspot.com


0 Response to "Which Of The Following Structures Undergoes The Most Physiological Changes During A Menstrual Cycle?"
Post a Comment